We are the only cosmetic suture company in the industry to have a $1,000,000 product liability policy. No other thread company can provide this level of security to and your patients.
Our leadership team is guided by Dr. Jonathan Sykes, MD, F.A.C.S. Dr. Sykes has performed over 20,000 surgical procedures and is an expert in rhinoplasty and aging related surgeries such as facelift, browlift, and eyelid lift surgery. His expertise is woven into the five core values of the Les Encres organization… SAFETY MINDED. SCIENCE BASED. EDUCATION POWERED. HEART DIRECTED. LEADERSHIP INSPIRED.
The purchase of Les Encres Cosmetic Threads is available only to Licensed Medical professionals.
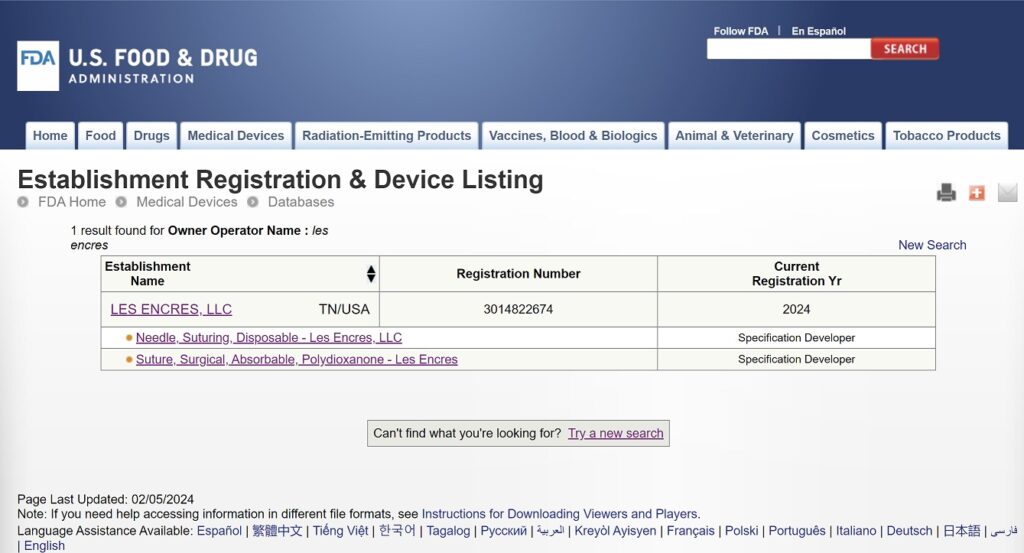
The sale of some Les Encres, LLC products may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies.
Below choose your NEW CUSTOMER WAIVER AGREEMENT, upon on boarding with Les Encres you will be able to purchase your threads using our ON-LINE ORDER FORM !
(CLICK OPTION BELOW)
NEW CUSTOMER AGREEMENT WAIVER – (Purchase of ONLY Threads – EXISTING THREAD USER – No Training Needed)
OR
NEW CUSTOMER AGREEMENT WAIVER – (Purchase of Threads and TRAINING Session Requested)